MEET EmboPore technology
EmboPore is a technology invented in the laboratory of Prof. Ofra Benny, the Hebrew University of Jerusalem. EmboPore technology has received the prestigious European Research Council grant.
TACE Limits
The Problem with Conventional TACE Therapy
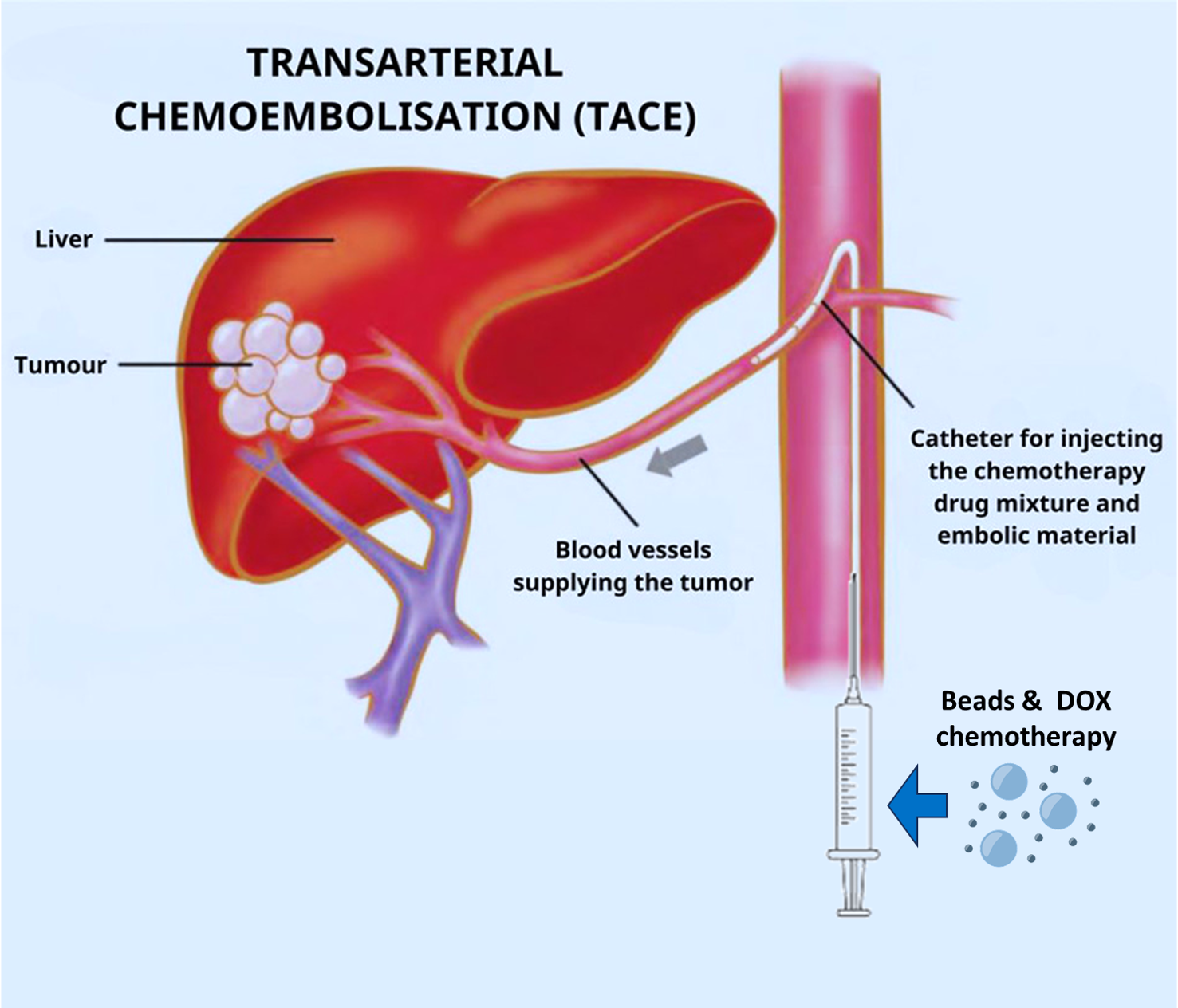
TACE (Trans-Arterial Chemo-Embolization) is the standard treatment for inoperable liver cancer. However, it presents major limitations.
Complete vessel embolization (causing Ischemia): Full vessel blockage while completely starving the tumor withholds most of the chemotherapeutic drugs from reaching its target
Immediate arterial blockage: short lived tumor drug exposure, while the rest is washed away
Short drug pulse & rapid ischemia: tumor exposure to upregulated pro-proliferation molecules
<span data-metadata=""><span data-buffer="">This results in a poor prognosis: only 20% 5-year survival.
Our Technology
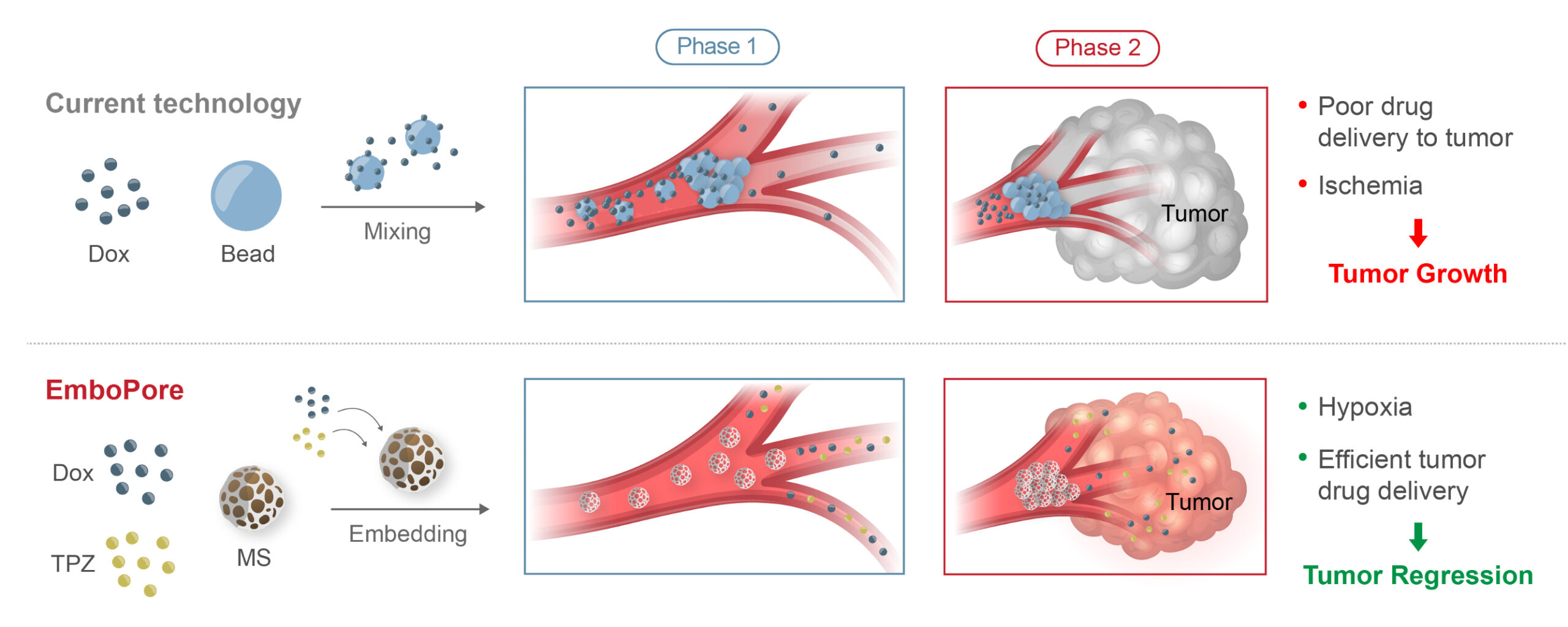
EMBOPORE - CONTROLLED DRUG RELEASE COMBINED WITH OPTIMAL ARTERY BLOCKAGE
hypoxic environment to enable tumor drug availability and enhance drug efficiency
Precise location
EmboPore injection utilizes TACE procedure to directly reach the tumor's feeding arteries
Optimal arterial blockage
Microspheres partially block blood flow, creating a hypoxic (oxygen-deprived) environment without full vessel occlusion.
Synergistic drug efficacy
Combined DOX & hypoxia-activated TPZ provide dual anti-cancer effect targeting different signaling pathways creating synergistic impact
Sustained Drug Release
The embedded drugs are gradually released over 14 days, maximizing therapeutic impact and improving patient outcomes.
Comparison TACE vs. EmboPore
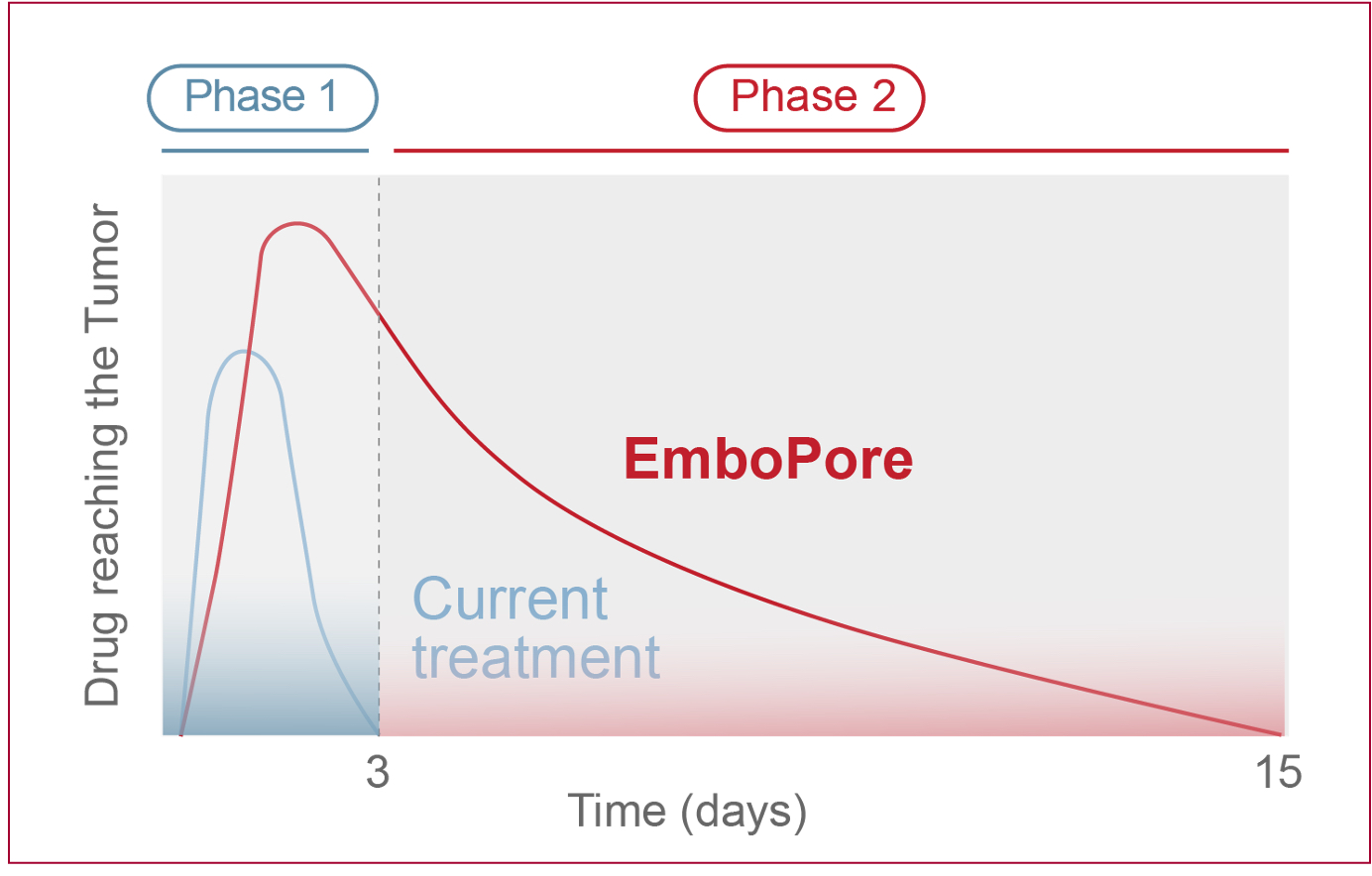
In Phase 1, TACE rapidly & completely blocks blood and drug flow, starving the tumor & enabling only a short-lived chemotherapeutic drug flow and poor drug delivery leading to rapid washout” to “resulting in residual drug washout. EmboPore allows partial blood flow to create a hypoxic environment for drug activation and initial high drug release
In Phase 2, TACE drug delivery is eliminated. EmboPore, however, ensures additional sustained drug release for up to 14 days, maximizing tumor drug exposure, elevating efficacy and promoting tumor regression.
Preclinical Results & Scientific Validation
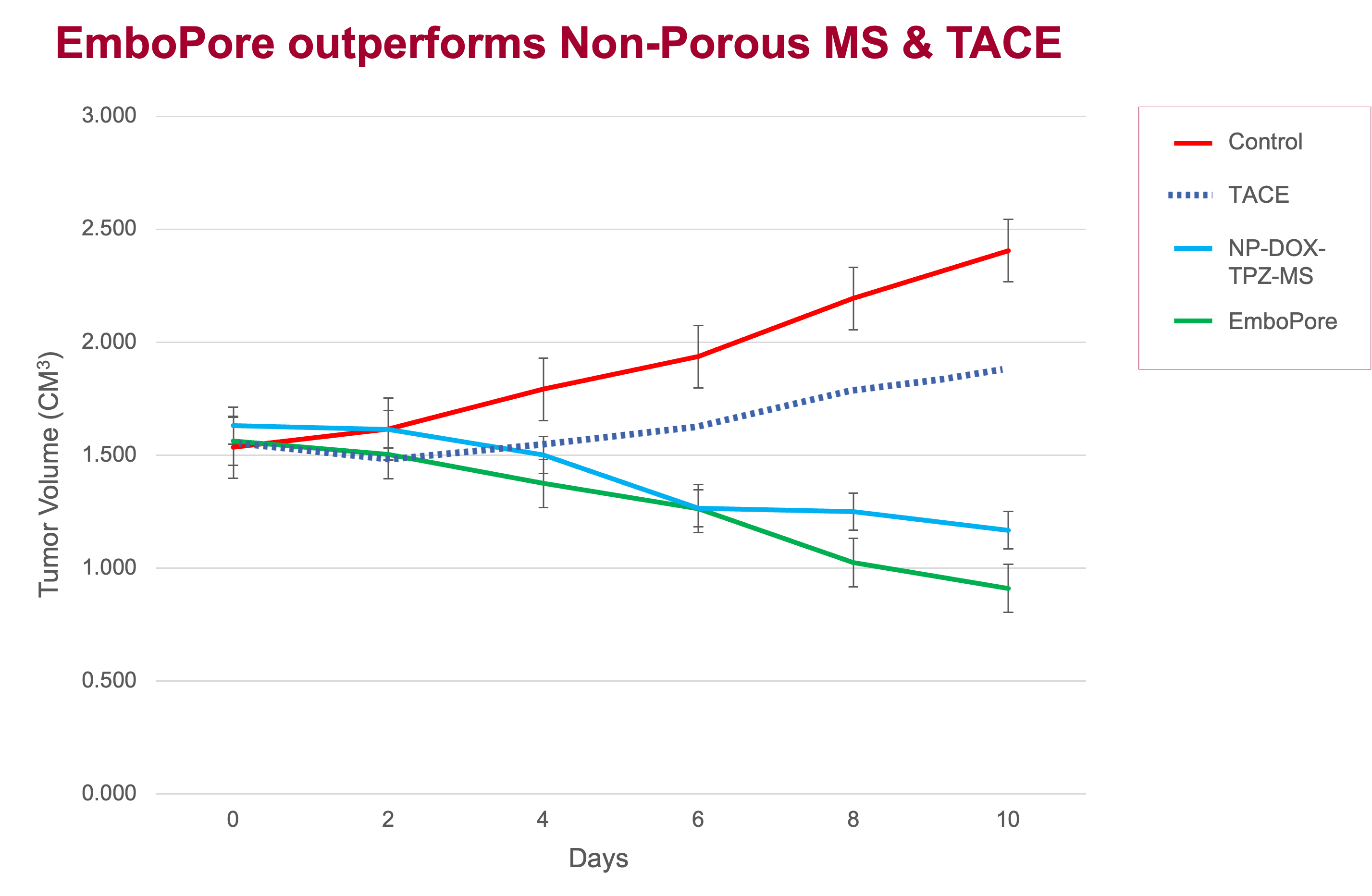
Strong Preclinical Evidence Supporting EmboPore
In an elaborate research study, including rat models, EmboPore significantly outperforms non-porous microspheres & TACE therapy, effectively reducing tumor volume.

Join the EmboPore Movement
With strong scientific backing, a clear market need, and a patent-pending breakthrough technology, EmboPore is positioned for success. Now is the time to invest.